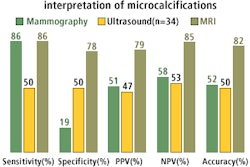
An October 16, 2002 meeting of the FDA Radiological Devices Panel of the Medical Devices Advisory will discuss, make recommendations, and vote on a premarket approval application for a device that produces a computerized thermal image of the breast of women recommended for biopsy.
FDA Radiological Devices panel to discuss breast thermal imaging
Sep 20, 2002
Latest in Breast
False-positive DBT findings differ between AI, radiologists
October 2, 2025
Women who skip first mammogram more likely to die of breast cancer
September 24, 2025
Study finds AI not cost-effective for breast cancer screening
September 24, 2025