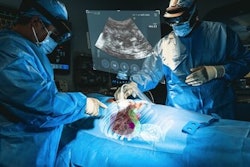
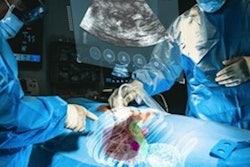
MediView has begun enrolling patients for a clinical study validating the use of its imaging and guidance augmented reality (AR) platform.
The multicenter study, "Clinical Evaluation of an Intra-procedural 3D Needle Guidance Platform for Performing Percutaneous Soft Tissue Tumor Biopsy as an Adjunct to Standard Image Guidance," will evaluate the procedural efficiency of MediView's XR90 holographic surgical navigation system as an adjunct to imaging modalities such as ultrasound and CT for percutaneous tumor biopsy procedures compared to imaging alone, MediView said.
The study aims to enroll 104 patients over the next 24 months at centers including New York-Presbyterian/Weill Cornell Medical Center and Weill Cornell Medicine, MedStar Georgetown University Hospital, and MedStar Washington Hospital Center, the Cleveland-based medtech firm added.