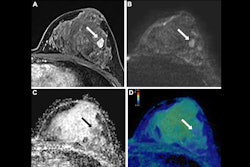
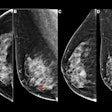
Abbreviated MRI and contrast-enhanced mammography (CEM) may be the best supplemental imaging methods for women with dense breasts, a study published May 21 in The Lancet found.
A team led by Fiona Gilbert, MD, from the University of Cambridge in England found that both modalities detected three times as many invasive cancers compared with automated breast ultrasound (ABUS). And cancers detected by MRI and CEM were found at half the size of those detected by ABUS.
“I think probably the most pleasing of our findings is that we now have a choice we can offer women with dense breast tissue,” Gilbert told AuntMinnie.
Since the initial publication of results from the Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, women with dense breasts have been recommended for supplemental MRI along with their regular mammography exams. However, conventional MRI has its logistical and access barriers that may hinder some patients from receiving their supplemental imaging.
The Breast screening Risk Adapted Imaging for Density (BRAID) study is a randomized controlled trial taking place at 10 U.K.-based breast screening sites. For the study, women ages 50 to 70 were independently assigned to either abbreviated MRI, ABUS, CEM, or full-field digital mammography (standard of care). The researchers included women who had negative mammograms and dense breasts.
Gilbert and colleagues reported interim results from the first round of supplemental imaging for the BRAID study. These interim results included data collected between 2019 and 2024 from 6,305 study participants who completed supplementary imaging. From the total, 2,130 underwent abbreviated MRI, 2,141 completed ABUS exams, and 2,035 underwent CEM.
Fiona Gilbert, MD, explains the results of the BRAID trial for women with dense breasts.
Abbreviated MRI and CEM had the highest cancer detection rates of the modalities included, with each also finding more invasive cancers than ABUS. However, ABUS led to lower recall and biopsy rates.
Comparison between supplemental breast imaging modalities in BRAID study | |||
Measure | ABUS | Abbreviated MRI | CEM |
Cancer detection rate (per 1,000 exams) | 4.2 | 17.4 | 19.2 |
Invasive cancer detection rate (per 1,000 exams) | 4.2 | 15 | 15.7 |
Recall rate | 4% | 9.7% | 9.7% |
Biopsy rate | 1.5% | 4.9% | 4.4% |
The team reported one case of extravasation in the abbreviated MRI arm, no adverse events in the ABUS arm, and 24 iodinated contrast reactions in the CEM arm. For the latter, this included 17 minor events, six moderate, one severe case, and three extravasations.
Gilbert said the abbreviated MRI results replicate those of the DENSE trial and that CEM can also match these results.
“In many respects, CEM is more easily delivered within breast units, because often MRI scanners are in the hospitals and it’s more difficult to get breast imaging slots for those machines,” she told AuntMinnie.
Gilbert said the team is looking at a more stratified approach to measure breast cancer risk for these women, which may include patient questionnaires and polygenic risk scoring via genetic testing. It is also working on follow-up data to measure potential interval cancers in the BRAID study.
Gilbert explains how radiologists can help better measure cancer risk from breast density.
What others are saying
The BRAID study results have caught the attention of leading breast imaging researchers, educators, and advocates.
Joann Pushkin and Wendie Berg, PhD, cofounders of DenseBreast-info.org, reiterated that a standard mammogram is not enough for women with dense breasts and that “the debate is over.”
“Denying women with dense breasts supplemental screening results in an unconscionable number of undetected cancers and, in the U.K., creates a potential 3,500 family tragedies in the form of late-stage diagnoses,” Pushkin told AuntMinnie.
“It is important that nearly 90% of the women in this trial were of otherwise average risk for breast cancer,” Berg said. “Increased cancer detection was accompanied by a nearly 10% recall rate for contrast methods. There was also a 1.2% rate of contrast reactions in the women receiving iodinated contrast for CEM. These factors need to be considered. CEM is not currently FDA-approved for screening.”
And Linda Moy, MD, from NYU Langone Health in New York, said that despite the tradeoffs seen in recall rates, the results mean good news for women with dense breasts.
“If women want to have supplemental screening, they should have either of the contrast imaging tests that are rapidly being provided at many facilities,” said Moy, who also serves as immediate past president of the Society of Breast Imaging and editor of the RSNA journal, Radiology.
The full study can be read here.