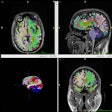
The U.S. Food and Drug Administration (FDA) has cleared Hyperfine's first update to its Optive AI software.
The update consists of a multi-direction diffusion-weighted imaging (DWI) sequence for its portable MRI system, Swoop system, the company said. The sequence acquires and averages signals in a similar way to a method used in high-field MRI scanners, to produce cleaner, more consistent images, according to Hyperfine.
The multi-direction DWI sequence is the first of a number of advancements Hyperfine plans to develop for Optive AI, it noted.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)