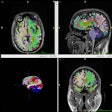
Eli Lilly has secured U.S. Food and Drug Administration (FDA) approval for a Kisunla label update that is expected to lower the incidence of amyloid-related imaging abnormalities with edema/effusion (ARIA-E).
Kisunla (donanemab) was approved in 2024 for the treatment of early Alzheimer's disease, including mild cognitive impairment or mild dementia. Safety warnings include that the drug can cause ARIAs, which are detected by MRI and present as temporary swelling in an area or areas of the brain.
The new dosing recommendation differs from the original dosing by shifting a single vial from the first dose to the third dose, which delivers the same amount of Kisunla by week 24. This results in lower rates of ARIA-E without compromising the drug's ability to reduce amyloid plaque, the company said.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)