Researchers have developed a strategy based on cardiac MRI that can identify patients at risk of sudden death due to dilated cardiomyopathy, according to a study published September 9 in Radiology.
The strategy leverages the prognostic value of a tool called late gadolinium enhancement (LGE) and offers a new quantitative versus qualitative approach for assessing patients, noted lead author Di Zhou, MD, of the Chinese Academy of Medical Sciences in Beijing, China, and colleagues.
“Our research demonstrates that a quantitative approach, specifically using a cutoff value of LGE of at least 7.2%, offers a more robust assessment of [sudden cardiac death] risk,” the group wrote.
Dilated cardiomyopathy causes the chambers of the heart to grow larger, and if left untreated, it can lead to sudden cardiac death. LGE on cardiac MRI is a powerful marker of myocardial fibrosis, or scarring, but has also shown potential based on visual reads for identifying vulnerable areas of the heart in patients with dilated cardiomyopathy, the researchers explained.
However, they noted that these qualitative assessments are limited, particularly when interpreting LGE in areas with intermediate signal intensity. Thus, in this study, the group evaluated the clinical performance of percentage LGE extent in predicting death in a group of 837 patients with the disease.
Participants (mean age, 46.6) were retrospectively enrolled from February 2012 to November 2019, as well as prospectively enrolled in a clinical trial from December 2019 to September 2021. The median follow-up was 58.3 months, and the primary endpoint was a composite of sudden cardiac death (SCD)-related events.
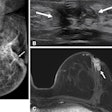
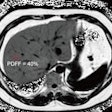
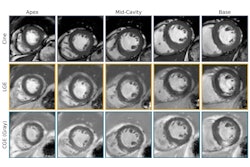
 (A) Example MRI studies in a 38-year-old male patient who experienced sudden cardiac death (SCD) 17 months after cardiac MRI. Late gadolinium enhancement (LGE) images show no visually detectable enhancement (top row). Native T1 mapping images show that the native myocardial T1 z score is 2.49 (middle row), and extracellular volume fraction (ECV) images show a quantitative ECV of 33.4% (bottom row). (B) Example MRI studies in a 46-year-old male patient without SCD events during the 106 months after cardiac MRI. LGE images show focal enhancement in the midwall with a 6.5% enhancement burden of left ventricular myocardium (top row), native T1 mapping images show that the myocardial T1 z-score is 0.1 (middle row), and ECV images show a quantitative ECV fraction of 26.9% (bottom row). RSNA
(A) Example MRI studies in a 38-year-old male patient who experienced sudden cardiac death (SCD) 17 months after cardiac MRI. Late gadolinium enhancement (LGE) images show no visually detectable enhancement (top row). Native T1 mapping images show that the native myocardial T1 z score is 2.49 (middle row), and extracellular volume fraction (ECV) images show a quantitative ECV of 33.4% (bottom row). (B) Example MRI studies in a 46-year-old male patient without SCD events during the 106 months after cardiac MRI. LGE images show focal enhancement in the midwall with a 6.5% enhancement burden of left ventricular myocardium (top row), native T1 mapping images show that the myocardial T1 z-score is 0.1 (middle row), and ECV images show a quantitative ECV fraction of 26.9% (bottom row). RSNA
According to the findings, a quantitative LGE threshold of at least 7.2% was a powerful independent predictor of SCD-related events (hazard ratio, 4.75, with 1 as reference) and demonstrated superior SCD risk stratification compared with qualitative distribution (adjusted C statistic: 0.783 vs. 0.732).
In addition, in patients at moderate risk -- defined as those with an LGE of less than 7.2% -- an extracellular volume fraction (ECV) threshold of 31.8% improved risk discrimination. The subgroup with a left ventricular ejection fraction of more than 35%, LGE of less than 7.2%, and ECV of less than 31.8% exhibited a low annual SCD event rate of 0.2%, the researchers reported.
By providing cutoff values for LGE and ECV, the research introduces a nuanced approach that enhances the predictive capability across clinical scenarios, the investigators wrote.
“The proposed comprehensive workflow empowers clinicians to implement personalized strategies, which may ultimately lead to improved patient outcomes in this vulnerable cohort,” they concluded.
In an accompanying editorial, Hajime Sakuma, MD, PhD, of Mie University in Tsu, Japan, and chairman of the Japanese Society of Cardiovascular Radiology, wrote that the proposed thresholds could allow clinicians to identify high-risk patients, but equally importantly, low-risk patients for whom implantable cardioverter-defibrillator therapy, for instance, is “absolutely unnecessary.”
“The results of this study are valuable because the proposed workflow integrating LGE, ECV, and LVEF thresholds is simple enough to be implemented in clinical practice,” Sakuma concluded.
The full study is available here.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)