Mammographic calcifications found in HER2-positive breast cancers impact treatment performance, according to research published September 9 in Radiology.
A team led by Eun Sook Ko, MD, PhD, from Sungkyunkwan University in Seoul, South Korea, found that breast calcifications lower the performance of radiologic complete response on MR imaging in predicting pathologic complete response. And this may be tied to residual ductal carcinoma in situ (DCIS).
“Although radiologic complete response at MRI remains valuable for predicting pathologic complete response, its lower positive predictive value [PPV] in patients with calcifications suggests a risk of underestimating residual disease,” Ko and colleagues wrote.
The increase in pathologic complete response rate observed with treatment regimens has raised questions regarding whether surgery is needed in women suspected of having a complete response.
However, HER2-positive breast cancer shows with mammographic calcifications in as many as 56% to about 65% of women. These calcifications often persist following neoadjuvant chemotherapy, even when MRI shows no residual lesions. This creates uncertainty about whether surgery is needed.
Ko and colleagues identified factors tied to pathologic complete response. They also evaluated whether mammographic calcifications affect the performance of radiologic complete response at MRI for predicting complete response in women with HER2-positive breast cancer. The women were treated with a regimen of trastuzumab and docetaxel alone, establishing docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP).
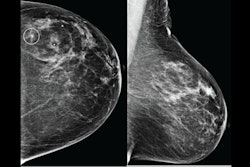
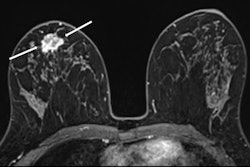
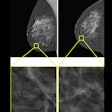
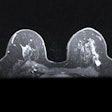
 Images of the right breast in a 63-year-old woman with estrogen receptor–low, HER2–positive breast cancer. (A) Mediolateral oblique mammogram obtained before neoadjuvant chemotherapy shows a 5-cm, irregular, spiculated, hyperdense mass (arrows) without calcification in the lower central breast. (B) Axial contrast-enhanced T1-weighted MRI scan obtained before chemotherapy shows two abutting malignant masses (arrows) in the lower central breast. (C) Mediolateral oblique mammogram after chemotherapy shows a decrease in tumor size, with only a biopsy marker (arrow) at the corresponding site. (D) Axial contrast-enhanced T1-weighted MRI scan after chemotherapy shows no residual enhancement in the tumor bed. This radiologic complete response corresponded with pathologic complete response, as determined via pathologic examination after breast-conserving surgery.RSNA
Images of the right breast in a 63-year-old woman with estrogen receptor–low, HER2–positive breast cancer. (A) Mediolateral oblique mammogram obtained before neoadjuvant chemotherapy shows a 5-cm, irregular, spiculated, hyperdense mass (arrows) without calcification in the lower central breast. (B) Axial contrast-enhanced T1-weighted MRI scan obtained before chemotherapy shows two abutting malignant masses (arrows) in the lower central breast. (C) Mediolateral oblique mammogram after chemotherapy shows a decrease in tumor size, with only a biopsy marker (arrow) at the corresponding site. (D) Axial contrast-enhanced T1-weighted MRI scan after chemotherapy shows no residual enhancement in the tumor bed. This radiologic complete response corresponded with pathologic complete response, as determined via pathologic examination after breast-conserving surgery.RSNA
The study included 732 women with an average age of 51.9, 474 of whom (64.8%) had calcifications. Also, 344 of the total women (47%) had radiologic complete response at MRI, and 301 (41.1%) had pathologic complete response.
The team identified the following factors that are associated with pathologic complete response: estrogen receptor-low subtype (odds ratio [OR], 3.07; p = 0.006), the hormone receptor-negative subtype (OR, 3.28; p < 0.001), absence of calcifications (OR, 1.65; p = 0.006), and radiologic complete response at MRI (OR, 7.33; p < 0.001).
Pathologic complete response rate, PPV of radiologic complete response at MRI in women with and without calcifications | |||
Measure | Women without calcifications | Women with calcifications | P-value |
Pathologic complete response rate | 48.4% | 37.1% | 0.003 |
PPV | 73.1% | 60% | 0.01 |
Finally, when the pathologic complete response definition was broadened from ypT0 to ypT0/Tis, the team reported no evidence of a difference in the complete response rate between women with and without calcifications (62% vs. 58.5%; p = 0.35).
The study authors highlighted that persistent calcification after chemotherapy may mean surgery is needed to eliminate DCIS.
“Considering these factors in surgical decision-making may lead to optimal treatment strategies and improve oncologic outcomes in patients with HER2-positive breast cancer,” they wrote.
In an accompanying editorial, Lars Grimm, MD, from Duke University in Durham, NC, wrote that the findings “shed light on the challenges for clinicians treating patients whose HER2-positive breast cancer includes calcifications.”
“The poor performance may shift the risk-benefit calculations for oncologists regarding offering [neoadjuvant chemotherapy], and it may prompt radiologists to recommend additional workup, including percutaneous biopsy,” Grimm wrote.
Read the full study here.