Sirona Medical has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the company's Sirona Advanced Imaging Suite.
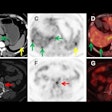
This clearance marks the company's first class II medical device designation. It expands Sirona's diagnostic imaging capabilities to include PET/CT support with quantitative standard uptake value analysis, image fusion, maximum intensity projection generation, and multiplanar reconstruction.
The company highlighted that with the FDA clearance, new functionality no longer requires complex, site-by-site installations or lengthy upgrade cycles. The suite's capabilities can be deployed at once to every user through the cloud.
Sirona Medical said that the Sirona Advanced Imaging Suite will begin rolling out to users as early as November, with immediate availability for existing customers participating in early-access programs.