Lantheus and GE HealthCare (GEHC) have inked a licensing agreement for GEHC to develop, manufacture, and commercialize Lantheus’ PET radiotracer F-18 piflufolastat in Japan.
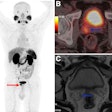
F-18 piflufolastat (Pylarify in the U.S.) is used for imaging prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer. The agreement includes the transfer of regulatory dossiers, manufacturing competencies, and technical support to enable GEHC to drive clinical development in Japan toward potential regulatory submissions and commercial launch, Lantheus said in a news release.
GEHC will draw on its manufacturing network and R&D expertise following its acquisition in March 2025 of Nihon Medi-Physics, a leading radiopharmaceutical company in Japan, Lantheus noted.