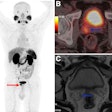
The U.S. Food and Drug Administration (FDA) has approved expanded indications for GE HealthCare’s (GEHC's) Vizamyl PET imaging agent for beta-amyloid detection.
The updated indications, which are effective immediately, allow the use of Vizamyl (flutemetamol F-18 injection) for quantification of amyloid, as well as enabling clinicians to use Vizamyl to monitor patient response to antiamyloid therapies.
GEHC noted in a statement that with the FDA lifting the restriction on using Vizamyl to monitor antiamyloid therapy, the imaging agent can now be used to assess whether the level of amyloid plaques has been reduced sufficiently for the therapy to be discontinued.
Other approved updates include permitting the use of Vizamyl for the selection of patients eligible for therapy, to predict the development or progression of dementia or other cognitive decline in Alzheimer’s disease, and to establish an Alzheimer’s disease diagnosis.