Genicular artery embolization (GAE) using mesenchymal stem cells appears effective for patients with bilateral knee osteoarthritis, Iranian researchers have reported.
The finding is from a feasibility and safety study in 30 patients with the disease and suggests the interventional radiology procedure could be a new treatment, noted lead author Hossein Ghanaati, MD, of Tehran University of Medical Sciences, and colleagues.
"This procedure may represent a new therapeutic avenue for patients with knee [osteoarthritis], given the established anti-inflammatory, immunomodulatory, and safety profiles of stem cell therapy," the group wrote. The study was published June 26 in the Journal of Vascular and Interventional Radiology.
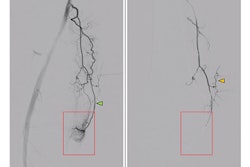
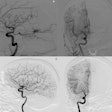
GAE is an emerging minimally invasive intervention for patients with painful knee osteoarthritis. The procedure involves using microcatheters to deliver embolic agents to small arteries in the knee to catheterize them and block the flow of blood. This reduces pain signals and provides relief, the authors explained.
In recent years, intra-articular cell therapy -- where stem cells are injected into the joint space -- has gained significant attention for treating osteoarthritis, offering hope for tissue repair, they added. Yet pain, inflammation, and adverse effects are reported in up to 67% of cases after the treatment, they noted.
In this study, the researchers aimed to evaluate the feasibility and safety of the genicular artery embolization procedure to deliver mesenchymal stem cells.
They first harvested the stem cells from umbilical cord tissue samples collected during cesarean sections. Genicular artery embolization was performed unilaterally in each subject using 70 million cells per injection. The cells were gently injected over 10 to 15 minutes. The researchers omitted postinjection fluoroscopic imaging to avoid any potential cell toxicity from radiation.
Technical success was defined by the successful delivery of cells. Clinical outcomes were assessed with the Visual Analog Scale (VAS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) before treatment and during a subsequent 12-month follow-up period.
According to the results, technical success was achieved in 100% of cases. The mean procedure time was 13.7 minutes. There were two grade A procedure-related adverse events (temporary skin discoloration) and no adverse events during the study follow-up period. Significant improvements were observed in WOMAC scores, from a mean of 29.6 prior to the procedure to 4.6 at 12 months (p < 0.001) and VAS scores, from a mean of 6.8 to 2 at 12 months (p < 0.001), the researchers reported.
"This study demonstrated a significant reduction in pain and clinical symptoms over 12 months, as evidenced by decreased WOMAC and VAS scores," the group wrote.
Ultimately, GAE is an emerging procedure and is evolving relative to other potential percutaneous therapies for knee osteoarthritis, such as genicular nerve ablation, the authors noted. This includes developing new embolic agents, they added.
"Investigators have and are actively reporting on different embolics for this application, including imipenem, lipiodol, and others. Stem cells may represent an additional novel option for embolics in this context," the researchers concluded.
The full study is available here.