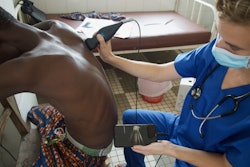
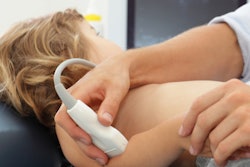
Vancouver, BC-based Sonic Incytes Medical has secured clearance from the U.S. Food and Drug Administration (FDA) for its point-of-care ultrasound (POCUS) elastography device, Velacur One.
Velacur One measures attenuation and liver stiffness using 3D S-WAVE, and features a new AI output measurement for liver fat specific to the device called Velacur Determined-Fat Fraction. These measurements aid in the management of chronic liver disease, including metabolic dysfunction-associated steatohepatitis and metabolic dysfunction–associated steatotic liver disease, Sonic Incytes noted.