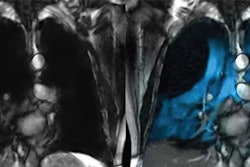
Polarean has submitted a phase III clinical trial protocol to the U.S. Food and Drug Administration (FDA) to support an expanded indication for its Xenoview contrast agent for lung MRI (hyperpolarized xenon-129).
The expanded indication would add quantitative gas-exchange imaging to the ventilation imaging indication for which Xenoview has already been approved.
The trial will evaluate the safety and diagnostic performance of Xenoview in assessing how effectively the lungs can transfer gas from the air into the bloodstream (i.e., pulmonary function) in comparison with current standard-of-care testing.
Quantifying and visualizing gas exchange is necessary across a range of chronic obstructive, interstitial, and pulmonary–vascular diseases, the firm noted in a statement. If approved, the indication would significantly broaden the utility of Xenoview in providing a more comprehensive noninvasive overview of lung function.
To finalize the study design, Polarean has formally requested feedback from the FDA under a Type C meeting. The FDA’s review of the proposed clinical trial should be completed by the fourth quarter of 2025, the company added.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)