Hermes Medical Solutions has secured U.S. Food and Drug Administration (FDA) clearance for the latest version of its flagship nuclear medicine imaging and dosimetry software, Hermia.
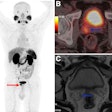
A major highlight of the latest version is the integration of the Centiloid scale, a standardized method for quantifying amyloid PET imaging results, the company said. The update now allows clinicians to do the following:
Interpret amyloid PET results across different tracers and protocols using a unified standard.
Enhance patient selection and treatment monitoring through improved comparability of quantitative imaging.
Optimize FDG-PET brain scan analysis with on-the-fly normalization method changes, supported by side-by-side 3D visualizations of deviation images.
Simultaneously display regions of both hyper- and hypometabolism in FDG-PET brain scans.
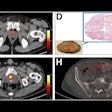
Analyze novel 3D digital SPECT/CT images with extended visualization and comparison capabilities.
In addition to the Centiloid scale integration, Hermia now supports loading CT-based anatomical regions for organ dosimetry. Users can integrate external segmentation software with automatic region delineation and alignment, which further streamlines the dosimetry workflow, Hermes said.