A patient showed a significant response in both primary and metastatic prostate cancer after first-line treatment with lutetium-177 (Lu-177) prostate-specific membrane antigen-617 (PSMA-617), researchers have reported.
The case involves a 54-year-old patient first diagnosed in 1996 and who continually declined androgen deprivation therapy (ADT), chemotherapy, and local therapy to the prostate gland, noted lead author Vinay K. Giri, MD, of Harvard Medical School in Boston, and colleagues.
“Following 2 cycles, he experienced a 95% reduction in PSA and improvement of urinary symptoms, as well as a durable and near-complete resolution of his prostate cancer primary lesion and nodal and osseous metastases,” the group wrote. The case was published September 25 in the Journal of Nuclear Medicine.
Lu-177 PSMA-617 (Pluvicto, Novartis) was approved in 2022 and is indicated for PSMA-expressing metastatic castration-resistant prostate cancer (mCRPC) after progression on ADT and androgen receptor pathway inhibitors (ARPIs) and can also be administered before chemotherapy, the authors explained. The use of Lu-177 PSMA-617 before ADT has been described in only a few published cases, but in none where patients did not receive any initial local therapy, they added.
In this case, after initial diagnosis, the patient opted for surveillance via PSA and MRI monitoring without additional biopsies or treatments because of concerns about side effects. By late 2023, his disease had progressed to metastatic hormone-sensitive prostate cancer (mHSPC), prompting the choice of off-label Lu-177 PSMA-617.
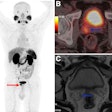
A follow-up PSMA-PET/CT scan performed after two doses demonstrated decreased avidity of the posterior left acetabular sclerotic metastasis (SUVmax decreased from 13 to 3.1), size and avidity of previously bulky retroperitoneal and bilateral pelvic lymphadenopathy (SUVmax decreased from 21–22 to 1.5–3.4), and radiotracer uptake in the posterior prostate (SUVmax decreased from 76 to 6).
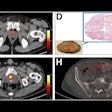
 Burden of PSMA-expressing prostate cancer before and after treatment with two cycles of Lu-177 PSMA-617. (A and B) Reduction in overall disease burden is evident after treatment. Avidity of patient’s primary prostate tumor and his osseous metastasis shown before (C) and after (D) treatment. Journal of Nuclear Medicine
Burden of PSMA-expressing prostate cancer before and after treatment with two cycles of Lu-177 PSMA-617. (A and B) Reduction in overall disease burden is evident after treatment. Avidity of patient’s primary prostate tumor and his osseous metastasis shown before (C) and after (D) treatment. Journal of Nuclear Medicine
After two doses, the patient declined any further treatment yet continued to have a prolonged PSA response 10 months from the start of treatment, the researchers wrote. In addition, he had not experienced any additional side effects from Lu-177 PSMA-617, including renal toxicity, and continued to have marked improvement in obstructive urinary symptoms.
“This treatment strategy adds to the expanding experience utilizing Lu-177 PSMA-617 earlier in the treatment of mHSPC and should prompt additional prospective trials to more fully evaluate its long-term efficacy and safety,” the researchers concluded.
In an accompanying editorial, Boris Hadaschik, MD, of University Hospital Essen in Germany, Silke Gillessen, MD, of Università della Svizzera Italiana in Lugano, Switzerland, and Oliver Sartor, MD, of the East Jefferson General Hospital Cancer Center in New Orleans, wrote that the case offers a limited but provocative contribution to the ongoing debate about treatment sequencing in prostate cancer, particularly for patients who decline standard therapy.
ADT comes with side effects, and continuous use can cause well-documented problems such loss of sexual function, loss of libido, osteoporotic fractures, fatigue, cardiovascular disease, and loss of muscle mass, they noted.
Nonetheless, ADT and ARPI are proven life-prolonging therapies and patients need to be well-informed that they may compromise their overall survival by avoiding or deferring them, they wrote.
“Although limited by its anecdotal nature and in a patient with metachronous disease, a setting with better prognosis per se, the case aligns with a growing interest in personalized, PSMA-targeted approaches and supports the need for prospective trials to test these strategies more broadly,” the authors concluded.
The full case report by Giri and colleagues can be found here.