A decade has passed since the first clinical experiences revealed the potential for prostate-specific membrane antigen (PSMA)-targeted paired diagnostic imaging and radioligand therapy for metastatic castration-resistant prostate cancer.
Since then, interest in the U.S. has grown in combining companion imaging with targeted systemic radiation therapy -- an approach recognized as theranostics (radiotheranostics, more specifically). This year marks expanded indications approved by the U.S. Food and Drug Administration (FDA) and a variety of cancer types with theranostics potential on the short-term horizon.
Precision oncology is the pursuit. One radiotheranostics insider estimates a $17 billion investment by biopharma alone, led by Novartis, Bristol Myers Squibb, and AstraZeneca. However, while radiopharmaceutical manufacturers exert pressure on the clinical workforce, private theranostic medicine practices are laying foundations, although at a slightly slower pace than probably hoped.
A decade has passed since the first clinical experiences revealed the potential for prostate-specific membrane antigen (PSMA)-targeted paired diagnostic imaging and radioligand therapy for metastatic castration-resistant prostate cancer.
Since then, interest in the U.S. has grown in combining companion imaging with targeted systemic radiation therapy -- an approach recognized as theranostics (radiotheranostics, more specifically). This year marks expanded indications approved by the U.S. Food and Drug Administration (FDA) and a variety of cancer types with theranostics potential on the short-term horizon.
Precision oncology is the pursuit. One radiotheranostics insider estimates a $17 billion investment by biopharma alone, led by Novartis, Bristol Myers Squibb, and AstraZeneca. However, while radiopharmaceutical manufacturers exert pressure on the clinical workforce, private theranostic medicine practices are laying foundations, although at a slightly slower pace than probably hoped.
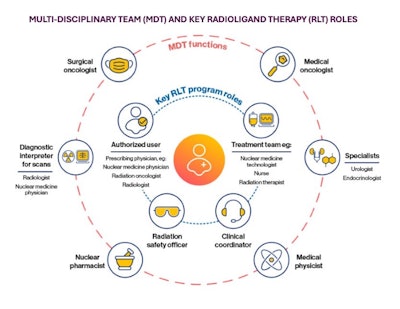
Regional capacity to fulfill demand for theranostic medicine using radioligand therapies (RLTs) depends heavily on radioactive materials (RAM) licensing, certified authorized users, and expertise in molecular and hybrid imaging and interpretation -- along with a specially trained care team and physicians, as shown in the graphic below.
 Jeremie Calais, MD, PhD, Ahmanson Translational Theranostics Division, David Geffen School of Medicine, University of California, Los Angeles (UCLA)
Jeremie Calais, MD, PhD, Ahmanson Translational Theranostics Division, David Geffen School of Medicine, University of California, Los Angeles (UCLA)
When The Lancet Oncology Commission explored the nuclear medicine workforce last year, the commission found gaps in the numbers of nuclear medicine physicians, medical physicists, nuclear medicine technologists, radiochemists, radiopharmacists, and nurses who are essential for the provision of a theranostics service.
A particular need for radiochemists and radiopharmacists was noted, even in high-income countries. Medical physicists were also uncommon in middle-income and high-income countries, according to the September 2024 report (The Lancet Oncology, September 30, 2024, Vol. 11, e545-e580).
Society of Nuclear Medicine and Molecular Imaging (SNMMI) President Jean-Luc Urbain, MD, PhD, has been highlighting this report in talks since November 2024. Coming up on one year, Urbain told AuntMinnie that the nuclear medicine and nuclear radiology physician workforce and other nuclear medicine professionals are trying to get ready for the availability of new theranostic compounds and radiopharmaceuticals for the disease's diagnosis and precise treatment.
"Up until about 2016 or 2017, 80% of what [nuclear medicine physicians] were doing was essentially imaging, like the radiologist," Urbain explained. "It's predicted that by 2030, we'll be similar to interventional radiologists, and we will be doing 60% to 70% of clinical work, so it's a huge paradigm shift that radiology was not ready for, and even the nuclear medicine field was not ready for, when basically the job market is limited."
Urbain continued, "For any field, when you decrease residency training programs and a new paradigm comes on board like radiopharmaceutical therapy, and more specifically theranostics, you don't have enough workforce to assume those developments on the clinical side and definitely not on the research side."
Radiation oncologists and interventional radiologists are positioned to help fill the gap, Urbain added.
Theranostics is practiced primarily in university or academic hospital settings, according to a preliminary SNMMI report. Notably, an SNMMI survey sent to 189 professionals and organizations asked whether nuclear medicine practices had implemented theranostics successfully.
Of 117 responses across nuclear medicine, industry, academia, and care teams, about 77% said yes and no: Some progress has been made, but there is still a lot of work to be done. About 10% of respondents indicated success; nearly 11% indicated that they have not been successful. Theranostics is emerging in a state of "splintered care" due to too few qualified medical professionals.
Imaging is well-established in terms of revenue generation, according to theranostics stakeholders whose inputs from a May 2025 summit will become the basis of a new SNMMI report highlighting industry needs. Radiopharmaceutical therapies are much more complicated, Urbain noted.
Theranostics models can involve practices taking on either the diagnostic imaging service and interpretation, or therapy administration alone. Full-service theranostics centers are beginning to emerge across the U.S. These centers have recognized advantages from linkages that make it easier to pivot.
Private practices expanding
AuntMinnie previously featured ARA Theranostics, a spinout division of ARA Diagnostic Imaging in Central Texas. Part of the Radiology Partners network, this practice maintains a large academic medical arm.
What eased ARA's transition to theranostics was having a RAM license, advanced interventional radiology (IR) suites, care coordinators on the IR side, and a multimodality imaging center, all under one roof.
Meanwhile, forming a nationwide network model of private practices, United Theranostics has begun expanding. AuntMinnie also featured this group last year.
The network grew out of training and education at the University of Maryland School of Medicine, with United Theranostics' first center in Maryland, then another in New Jersey, and one in New Mexico. In February, the group secured $15 million for expansion to an anticipated eight centers by year's end.
These nuclear oncology practices offer FDA-approved radiopharmaceutical therapies, each is equipped with PET/CT and SPECT/CT for advanced molecular imaging, and the network is staffed to conduct clinical trials for new radiopharmaceutical drugs under investigation and first-in-human trials.
Certified authorized users administer radiopharmaceutical therapy, according to United Theranostics enterprise Medical Director Munir Ghesani, MD. Staffing includes physicians, nuclear medicine technologists, patient care coordinators, and clinical trial staff as well.
Munir Ghesani, MD, PhD, United Theranostics chief medical officer and medical director of the Princeton, NJ, clinic since June 2024, discusses staffing challenges, partnership with the medical oncology community, plus what he is watching for in 2026.
About half of United Theranostics' procedures involve clinical trials, said Ghesani, who maintains academic ties with Mount Sinai Health System in New York, where he served as chief of nuclear medicine.
Likewise, nuclear oncologist Ashok Muthu Krishnan, MD, said he also maintains his academic affiliation as a professor of radiology at the University of Pittsburgh. Muthu Krishnan has a background in nuclear radiology, molecular imaging, nuclear medicine, and targeted radioactive therapy at University of Pittsburgh Medical Center.
Muthu Krishnan relocated to South Florida to establish a private nuclear medicine practice specializing in molecular imaging and theranostics procedures. For AuntMinnie, he explained how his practice, Florida Theranostics, started -- with radioligand therapy only -- but is growing to provide PET/CT and SPECT/CT scanning services to referring neurologists and support for clinical trials.
Ashok Muthu Krishnan, MD, introduces Florida Theranostics and explains how the practice began with staffing, next steps, and the need for nuclear medicine community physicians to open their own private practices. Plus, Muthu Krishnan explains his role as "nuclear oncologist," a term now formally recognized by the SNMMI.
Although there is currently increasing interest in amyloid PET imaging, 70% to 80% of Florida Theranostics services still cover prostate cancer patients and urologists with a combination of PET/CT -- and more recently SPECT/CT -- and radioligand therapy administration, Muthu Krishnan said.
Academic centers
While community-based, independent theranostics clinics such as Muthu Krishnan's and Ghesani's are coming online, academic medical center nuclear medicine services still drive most nuclear medicine and radiotheranostic procedural volume, according to the SNMMI.
UCLA Health was one of the first sites in the U.S. to participate in a phase II clinical trial in 2017 to test the safety and efficacy of the lutetium-177 (Lu-177)-PSMA radionuclide therapy now known as Pluvicto (Novartis).
Radiation safety, logistics, and clinical specialists designed UCLA's Outpatient Theranostics Center with eight infusion chairs, three nurses and one nurse practitioner, two nuclear medicine technologists, two radiation safety specialists, and two physicians.
The center opened in 2024 and was built for a capacity of 80 transfusions per week (4,000 treatment cycles per year), as well as the multidisciplinary team required for rising clinical trial demand, according to Jeremie Calais, MD, PhD, certified principal investigator and director of the clinical research program in UCLA Health's Ahmanson Translational Theranostics Division.
Aiding the practice of theranostics, UCLA maintains a biomedical cyclotron facility and radiochemistry "hot" laboratories that support both basic research investigations and patient imaging, as well as supply a variety of radiopharmaceuticals.
Clinical trial outlook
As 2025 begins to wind down, large industry-sponsored theranostic trials are designed around potential new applications, such as using radioligand therapy to treat prostate cancer and neuroendocrine tumors at an earlier stage. Others are comparing radiopharmaceutical treatments to other standard of care options such as chemotherapy -- results of which are eagerly awaited, Calais told AuntMinnie.
Phase III randomized trials Calais highlighted include the near-term PSMAAddition (NCT04720157). This international, prospective trial includes 1,145 patients with metastatic hormone-sensitive prostate cancer (mHSPC) and compares Lu-177-PSMA-617 plus standard of care (SOC) to androgen receptor pathway inhibitors and androgen deprivation therapy SOC alone.
Further out, STAMPEDE-2 (NCT06320067) will compare three new treatments, including stereotactic ablative radiotherapy (SABR), with the standard of care. Hospitals across the U.K. are involved, with an enrollment target of approximately 8,000.
In addition, PEACE-6 Poor Responders (NCT06496581) will investigate Lu-177 PSMA-617 when administered on top of the ongoing standard systemic treatment compared to standard systemic treatment alone in an estimated study population of 500.
Oligometastatic cancer in biochemical recurrence and dosing modulation trials are also focus areas, Calais added.
Moreover, molecular imaging targets have expanded well beyond PSMA and somatostatin receptor 2 (SSTR2) to human epidermal growth factor receptor 2 (HER2), fibroblast activation protein (FAP), [Glypican-3] GPC3, [carbonic anhydrase IX] CA-IX, [gastrin-releasing peptide receptor] GRPR, or poly (ADP-ribose) polymerase 1 (PARP-1), Calais said.
To manage clinical research trials at UCLA, it takes a team of at least 10, Calais explained. Through 2022, their work largely involved PSMA-PET imaging trials, initiating the new Pluvicto experience, and new targets like FAP or CA-IX.
However, industry phase II/III trials are now bringing new components and more requirements, according to Calais, whose clinical trials leadership is certified by the Association of Clinical Research Professionals (ACRP).
Jeremie Calais, MD, PhD, certified principal investigator and director of the clinical research program in UCLA Health's Ahmanson Translational Theranostics Division, reflects on this new era of theranostics clinical trials.
There are a variety of payment and reimbursement models, which create different incentives for different medical departments inside and outside of academic medical centers. And while the field of theranostics is taking off in some ways, early adopters and stakeholders are learning as they go, according to Urbain.
"Nuclear medicine, because of its background over the past 25 or 30 years in imaging, is somewhat struggling switching to a clinical environment," Urbain explained in an interview with AuntMinnie. "Theranostics is a good challenge because it will force us to have multidisciplinary collaborations and operations in order to optimize the treatment of these patients."
SNMMI President Jean-Luc Urbain, MD, PhD, discusses what the society knows about nuclear medicine needs in the U.S., utilization of PET/CT, and the drive for more SPECT/CT, as well as the push of industry to roll out more clinical trials.
This article is Part 6 of the series: The rise of theranostics. Read Part 5 here.

