A PET radiotracer can produce high-quality, real-time images of brain inflammation, suggest findings presented June 23 at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
In his presentation, Jiahui Chen, PhD, from Emory University in Atlanta, Georgia, discussed his team’s research showing that their radiotracer using dynamic PET imaging (F-18 PDE-1905) outperformed a translocator protein (TSPO)-specific tracer in a mouse model.
“For patients, this could mean earlier and more accurate diagnoses, better tracking of treatment effectiveness, and more personalized therapies based on direct measures of neuroinflammation,” Chen said.
Neuroinflammation is the immune system’s natural response to pathological conditions, including infection, toxin accumulation, and trauma in the central nervous system. This drives the progression of neurodegenerative diseases and psychiatric disorders such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS).
Phosphodiesterase 4B (PDE4B) is a subtype of the PDE4 enzyme family and has a role in the signal transduction of inflammatory factors. Previous studies suggest that upregulation of this enzyme occurs in response to neuroinflammatory conditions.
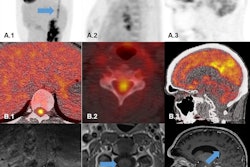
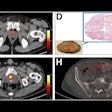
Chen and colleagues studied the potential of F-18 PDE-1905 as a novel PET tracer that focuses on PDE4B. They induced a neuroinflammation mouse model via intracerebroventricular injections of lipopolysaccharide (LPS). The team also conducted dynamic PET imaging for 60 minutes with F-18 PDE-1905 and a TSPO-specific tracer F-18 D2-LW223 for comparison.
 SNMMI
SNMMI
Bioinformatics analysis showed higher PDE4B levels in both Parkinson’s disease and MS patients and in corresponding mouse models. The team successfully established its neuroinflammation mouse model induced by LPS within 72 hours.
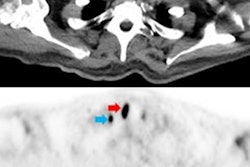
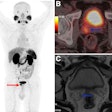
PET imaging showed significantly higher uptake of F-18 PDE-1905 in the brains of diseased mice compared to healthy controls. This means increased tracer activity in neuroinflammatory conditions. The team also found that F-18 PDE-1905 showed superior image quality and greater brain distribution compared to F-18 D2-LW223.
Western blot analysis confirmed the PET imaging findings, with PDE4B protein expression correlating strongly with tracer uptake (r = 0.92).
“By directly targeting PDE4B, F-18 PDE-1905 provides a more specific and upstream view of microglial activation, an early and critical factor in the progression of many neurological diseases,” Chen said in a prepared statement.
Chen highlighted the findings as valuable for studying neuroinflammatory processes. He added that future studies are needed to validate the role of microglia activation and its correlation with PDE4B.
“Ultimately, F-18 PDE-1905 has the potential to drive a major shift toward precision-guided care in neurodegenerative disorders,” Chen said.