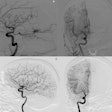
Microbot Medical has secured clearance from the U.S. Food and Drug Administration (FDA) for its endovascular robotic system, Liberty.
Liberty is a single-use, remotely operated robotic system for peripheral endovascular procedures, the company said. A clinical study of the device found 100% success in the robotic navigation to target, zero device-related adverse events, and a 92% relative reduction in radiation exposure for physicians, according to Microbot Medical.