4DMedical has announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its CT:VQ noncontrast, ventilation-perfusion imaging system.
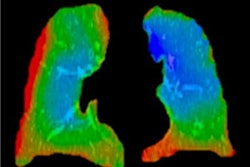
The system converts standard, noncontrast chest CTs into quantitative, lobar ventilation (V), and perfusion (Q) maps and is delivered as software-as-a-service (SaaS). Integrated with routine radiology workflows (DICOM-based, PACS reporting), the system brings functional lung imaging to sites without nuclear medicine capacity, according to the firm.
Clinical validation for CT:VQ included quantitative performance testing against SPECT, expert reader studies, and real-world cases across multiple lung conditions, the company said, noting favorable reimbursement conditions under the U.S. Centers for Medicare and Medicaid Services (CMS) in addition to existing reimbursement for the underlying chest CT.
In its announcement, 4DMedical said more than one million nuclear V/Q scans are performed annually in the U.S.