Taking global lung features into consideration on low-dose CT (LDCT) -- along with nodule characteristics -- appears to improve long-term lung cancer risk prediction, researchers have found.
Integrating these features into a lung cancer prediction model "addresses key limitations of existing approaches, such as overreliance on nodule size and the inability to predict cancers in negative screening populations," noted a team led by study co-authors Chengtin Lin, PhD, of Zhejiang Cancer Hospital in Hangzhou, China, and Weixiong Tan, PhD, of Beijing Deepwise & League of PHD Technology in Beijing. The group's results were published October 21 in Radiology.
Lung cancer screening with LDCT relies on characterizing nodules using the Lung-RADS framework and doesn't necessarily consider global lung features -- a strategy that may underestimate long-term cancer risk, the group explained. The study authors wrote that "age and smoking history are the primary factors used to identify individuals at high risk of lung cancer and nodule characteristics, such as density, size, and growth, are primarily considered to guide management," and cited one study that showed that considering these factors alone translated to a positive predictive value (PPV) of only 3.8%.
"Such a low PPV can lead to overdiagnosis or excessive follow-up," they noted.
Considering larger lung abnormalities such as chronic inflammation, emphysema, or pulmonary fibrosis could improve lung cancer risk prediction, Lin and colleagues hypothesized. They investigated any additional prognostic value conferred by including these characteristics via a study that used a three-year lung cancer prediction model, ScreenLungNet.
ScreenLungNet was developed using LDCT data from 19,869 individuals in the Wenling 2019 to 2020 screening cohort in Zhejiang Province, China, as well as CT data from 1,802 patients from Zhejiang Cancer Hospital between 2017 and 2023. Lin's team identified nodule characteristics taken from malignancy scores and global lung features and integrated them into the model. The group compared ScreenLungNet's performance to Lung-RADs using National Lung Screening Trial (NLST) data (14,966 patients), evaluating the area under the receiver operating characteristic curve (AUC), accuracy, specificity, sensitivity, positive predictive value (PPV), and negative predictive value.
Overall, they found that ScreenLungNet outperformed Lung-RADS in the NLST cohort for three-year lung cancer risk prediction, with an AUC of 0.93 (p < 0.001). The group also reported the following:
ScreenLungNet performance in the NLST cohort | |
Measure | Findings* |
| AUC | 0.93 |
| Accuracy | 94.8% |
| Sensitivity | 84.9% |
| Specificity | 95.2% |
| Positive predictive value | 44% |
| Negative predictive value | 99.3% |
| *All p < 0.02 | |
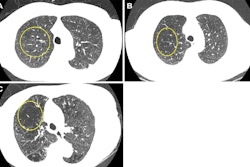
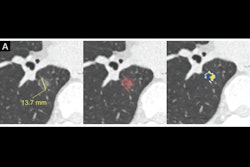
 Case-based visualization of model predictions and heatmap analysis for lung cancer risk assessment in baseline nodule-free and nodule-existing examples. (A-D) Images in a 68-year-old man with a 96 pack-year smoking history and no history of respiratory disease. (A) There are no visible nodules in the left lower lobe at baseline chest low-dose CT. (B) Corresponding baseline heatmaps from the peripheral lung parenchyma where a tumor subsequently developed. Regions highlighted in red indicate areas with a stronger positive contribution to the predicted outcome, whereas regions in blue indicate areas with minimal contribution (ScreenLungNet risk 0.77). (C) Lung window and (D) contrast-enhanced mediastinal window images obtained 14.1 months after the baseline scan reveal a mass adjacent to the left lower hilar region (arrow). Subsequent bronchoscopy and biopsy confirmed the diagnosis of squamous cell carcinoma. (E-G) Images in a 63-year-old woman without a smoking history or respiratory disease. (E) Baseline chest low-dose CT image shows a solid nodule with a mean diameter of 7.5 mm in the left lower lobe (Lung CT Screening Reporting and Data System 3). (F) Corresponding baseline heatmap shows the model’s focus on the nodule and surrounding features (ScreenLungNet risk 0.98). (G) Follow-up images obtained 5.1 months after the baseline scan reveals nodule growth to a mean diameter of 7.8 mm. Subsequent surgical pathologic examination confirmed the diagnosis of mucinous adenocarcinoma. Images and caption courtesy of the RSNA.
Case-based visualization of model predictions and heatmap analysis for lung cancer risk assessment in baseline nodule-free and nodule-existing examples. (A-D) Images in a 68-year-old man with a 96 pack-year smoking history and no history of respiratory disease. (A) There are no visible nodules in the left lower lobe at baseline chest low-dose CT. (B) Corresponding baseline heatmaps from the peripheral lung parenchyma where a tumor subsequently developed. Regions highlighted in red indicate areas with a stronger positive contribution to the predicted outcome, whereas regions in blue indicate areas with minimal contribution (ScreenLungNet risk 0.77). (C) Lung window and (D) contrast-enhanced mediastinal window images obtained 14.1 months after the baseline scan reveal a mass adjacent to the left lower hilar region (arrow). Subsequent bronchoscopy and biopsy confirmed the diagnosis of squamous cell carcinoma. (E-G) Images in a 63-year-old woman without a smoking history or respiratory disease. (E) Baseline chest low-dose CT image shows a solid nodule with a mean diameter of 7.5 mm in the left lower lobe (Lung CT Screening Reporting and Data System 3). (F) Corresponding baseline heatmap shows the model’s focus on the nodule and surrounding features (ScreenLungNet risk 0.98). (G) Follow-up images obtained 5.1 months after the baseline scan reveals nodule growth to a mean diameter of 7.8 mm. Subsequent surgical pathologic examination confirmed the diagnosis of mucinous adenocarcinoma. Images and caption courtesy of the RSNA.
The bottom line? ScreenLungNet shows promise as a useful way to improve lung cancer risk prediction, according to the authors.
"Its strong performance across multiple cohorts demonstrates its potential to provide accurate long-term lung cancer assessment in diverse populations," they concluded.
In an accompanying editorial, Pedro Staziaki, MD, agreed. Staziaki is at the University of Vermont Medical Center in Burlington.
"In the context of rising air pollution, expanding city skylines (both vertically and horizontally), and an aging population, lung cancer prevention will remain a leading cause of mortality despite decreasing smoking rates," he wrote. "Models such as those published by Lin and Tan et al, which can assess the lungs globally to capture the full complexity of this remarkable organ, will play a crucial role in the coming years."
The complete study can be found here.